So many baking powders…
Ah, baking powder, that essential ingredient in most of what are commonly known as quick breads, including: pancakes, biscuits, cakes,
 muffins, cookies and pie crusts. A recent perusal of the baking isle at my local grocer turned up no less than four baking powder brands all with different chemical formulations. Some were labeled ‘double acting’ and some boasted of being ‘aluminum free.’
muffins, cookies and pie crusts. A recent perusal of the baking isle at my local grocer turned up no less than four baking powder brands all with different chemical formulations. Some were labeled ‘double acting’ and some boasted of being ‘aluminum free.’
Of course, baking powders are not the only way to leaven breads without yeast: there is baking soda in combination with an acid ingredient, such as buttermilk or cream of tartar; steam from liquids in the batter; and the foam from whipped egg whites. But the introduction of baking powder into the mix to provide leavening is convenient, consistent, and simple.
Baking powder works by producing bubbles of carbon dioxide when the main ingredient(s), come into contact with an acid through the introduction of water. Most baking powders include an ingredient, such as corn starch, to absorb moisture and preserve
 the strength of the formula. Some baking powders are double acting meaning they contain two acids; one that reacts with the introduction of water, and one that reacts in the presence of heat after the batter has been placed in a hot oven for baking. This results with an end product that is lighter and fluffier than that made with a single acting baking powder. Not only too little, but too much baking powder can result in quick breads that do not rise as expected.
the strength of the formula. Some baking powders are double acting meaning they contain two acids; one that reacts with the introduction of water, and one that reacts in the presence of heat after the batter has been placed in a hot oven for baking. This results with an end product that is lighter and fluffier than that made with a single acting baking powder. Not only too little, but too much baking powder can result in quick breads that do not rise as expected.
Three companies produce the majority of baking powders distributed to grocery stores in the U. S.: Clabber Girl Corporation; Kraft Foods; and a newcomer, ACH Food Companies, Inc., also the maker of ARGO Food Starch and Fleischmann’s Yeast. All of the major baking powders are double acting, but not all are the same or produce the same results.
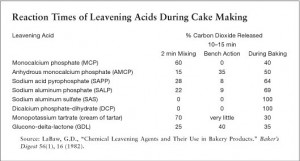
As stated before, some acids react immediately with the introduction of water, and some react when heated in the oven, but not all react with equal intensity. Some react with varying degrees at different stages of the process: mixing, baking, and even the time between the two which is described by Shirley Corriher as “bench action.”

In Harold McGee’s book “On Food and Cooking: The Science and Lore of the Kitchen” he offers this comparison of the reaction times of leavening acids: Immediately during mixing – Cream of Tarter (Tartaric Acid) and Monocalcium Phosphate; Slow release after mixing – Sodium Aluminum Pyrophosphate; Slow Release and Heat Activated – Sodium Aluminum Sulfate; Heat-Activated, Early In Cooking – Sodium Aluminum Phosphate and Dimagnesium Phosphate; and Heat-Activated, Late In Cooking – Dicalcium Phosphate Dihydrate.
Shirley Corriher offers this chart comparison, which is also helpful.
click on the chart to enlarge
It is extremely important to thoroughly mix chemical leaveners into the dry ingredients before the introduction of water. The introduction of water not only starts the reaction with some acids in the baking powder, but too much mixing can result in lessening the effects of carbon dioxide production in the batter through its dissipation from handling as well as produce an excess of gluten.
At this point I am reminded of a story by Alton Brown against handling biscuit dough too much, which is included in his book “I’m
 Just Here For More Food” (pp. 6-7). For years he had tried to “clone the tender little jewels of goodness that came out of her (his grandmother’s) oven.” He tried everything from copying her recipe to checking the variables of her kitchen: elevation, weather, temperature, even the calibration of her oven, all to no avail. It wasn’t until he closely observed her preparing the biscuits that he realized what he had been missing. “I watched her remove her rings, slowly twisting them over her arthritic knuckles…a ritual she undertook whenever she thought she might get her hands dirty. Since her hands were always their stiffest in the morning she rarely made biscuits for breakfast because…hey, wait a minute! The very affliction that caused her so much pain was also the secret to her biscuits. Because she could barely bend her fingers she handled the dough without really kneading it at(sic) all. She simply patted it. This is a small detail, yes…but in the end it’s the detail that made all the difference in the world.”
Just Here For More Food” (pp. 6-7). For years he had tried to “clone the tender little jewels of goodness that came out of her (his grandmother’s) oven.” He tried everything from copying her recipe to checking the variables of her kitchen: elevation, weather, temperature, even the calibration of her oven, all to no avail. It wasn’t until he closely observed her preparing the biscuits that he realized what he had been missing. “I watched her remove her rings, slowly twisting them over her arthritic knuckles…a ritual she undertook whenever she thought she might get her hands dirty. Since her hands were always their stiffest in the morning she rarely made biscuits for breakfast because…hey, wait a minute! The very affliction that caused her so much pain was also the secret to her biscuits. Because she could barely bend her fingers she handled the dough without really kneading it at(sic) all. She simply patted it. This is a small detail, yes…but in the end it’s the detail that made all the difference in the world.”
So which baking powder to use? My answer – ‘it depends.’ One obvious option is to use an aluminum free formulation which, until ARGO, was essentially only Rumford. However, even though Rumford is labeled as ‘double acting’ you will see from the chart above that Monocalcium Phosphate is not as effective a double acting agent as some other choices because it releases 60% of its carbon dioxide during mixing leaving only 40% for during baking.

Additionally, all the major baking powders I investigated which are available in the U. S. are certified Kosher. So, to determine which one (or ones) are best for your kitchen, my advice to you is to experiment with the different formulations to see which produces the results you are looking for. I would also suggest you try different baking powder formulations for different products. Please let me know your results.
Of course if you aren’t interested in experimenting, that’s fine too. You will be safe with just about whichever baking powder you choose as long as they are all of high quality. Just be sure to store them properly, in tightly sealed containers, and away from moisture.

Pingback: Baking Powder Experiment – Biscuits | Lukewarm Legumes()
Pingback: Jamaican Festival – the bread that is fun to eat | Lukewarm Legumes()